
20/06/2023
Large Molecule
Biologics and Generics have key differences that are important to understand. The pharmaceutical industry’s tango between innovation and affordability is one of the biggest challenges of the industry. Biologics have a unique complexity. Their steps are choreographed intricately to target and treat diseases previously thought of as untreatable. Across from them, generics waltz in with cost-effective replicas – aiming to perform for a budget-conscious audience. In this dynamic theater, biologics and generics compete for a spot in the future of medicine. Keep reading to examine what is at stake for the future of both business and healthcare.
Biologics
Biologics have been changing the medical landscape since their discovery in the 1970s. Many chronic illnesses have biologics to thank for treatment options. Treatments for illnesses like Crohn’s disease, Diabetes, Psoriasis, Arthritis, and breast cancer. Biologics have played the best role in attacking autoimmune diseases due to their ability to directly target the molecular pathophysiology or the ‘choreography’ of the disease.
Generics
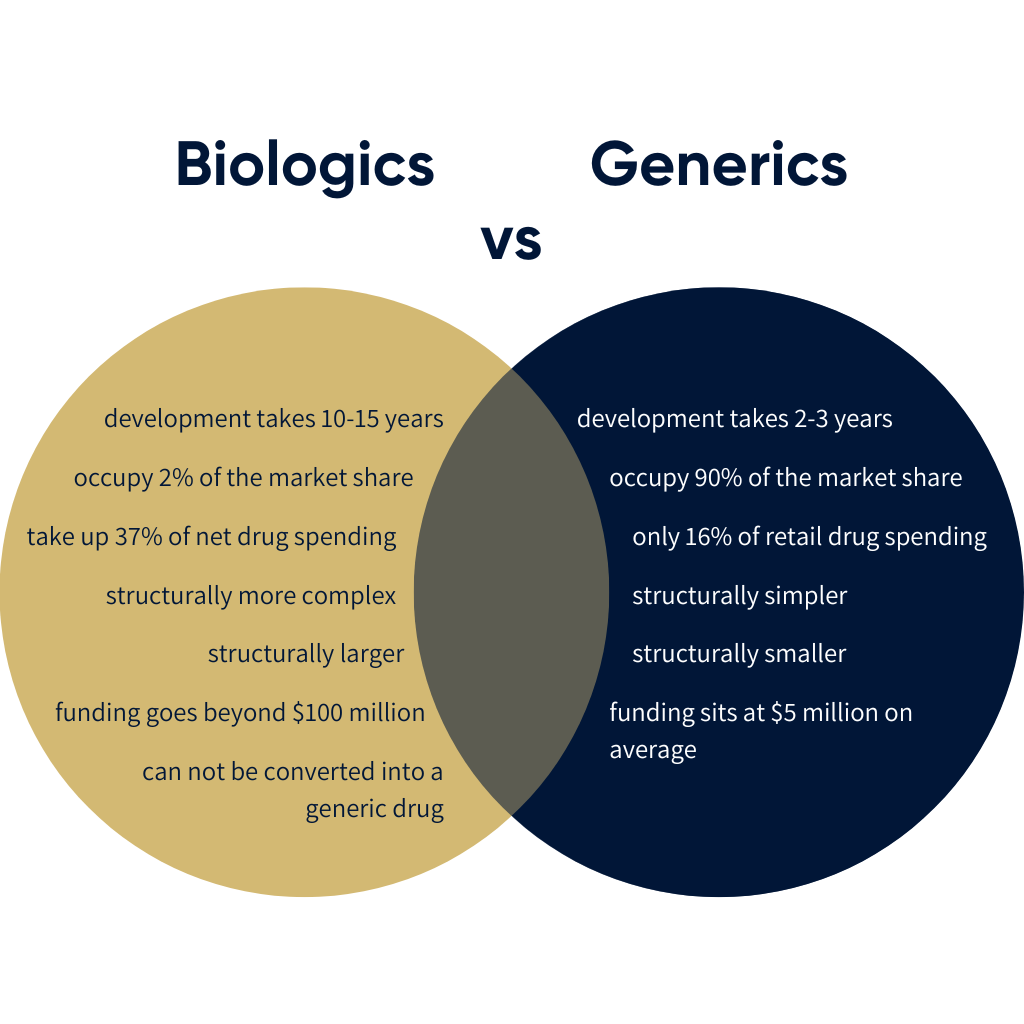
The key difference between generics and biologics is that while generics are structurally based in a concoction of chemicals, biologics find their protein structures in living cells. Their structural makeup is vastly different. Biologics are hundreds of times larger and more complex than Generics. Generics are also significantly cheaper to manufacture, and take 2-3 years to develop and test. Compare that to biologics which requires deeper pockets. Funding ranges beyond $100 million for research and development (R&D). It takes 5 to 9 years to develop and test, this is all before the manufacturing process has started. Generics have an upper hand on Biologics since generics are designed to replicate the effects of ‘name brand’ drugs after their patent expires – in essence, identical knockoffs brought down in price due to lower R&D costs than the company that initially bore those costs.
Biologics in Business
New research shows evidence of R&D costs for new medicines rising with time, which is to be expected. These studies show that R&D costs assume 50-58% of the budget per medicine. Studies also show that biologics are not performing as well as generics yet in relation to annual revenue, with a Forbes study citing biologic drugs accounting for a measly 2% of all U.S. prescriptions, but a massive 37% of net drug spending. These are not shocking statistics since biologics is a new field of study, compared to generics holding 70 years operating over biologics, but it leaves questions surrounding the future profitability of biologics.
Biologics may produce extraordinary results for chronic autoimmune disordered patients, but are biosimilars profitable? Considering the R&D costs for biosimilars, it is clear that the breakeven point for biotech companies is harder to reach than for small molecule drug companies. However, R&D costs are not the biggest risks that biotechnology firms are facing. Currently, the biggest obstacle for new biosimilars and biobetters are existing firms that have occupied a monopoly of the market. This type of monopoly is allowed under the patent laws to allow the original company that developed the drug to recover profits from R&D before competition swoops in.
Another challenge is simplifying the biologics treatment process for patients. Doctors must learn how to effectively simplify, communicate, and teach patients the right medical literacy to make informed decisions about their treatment plans. There are other obstacles to seeing real change in the industry now. As patients discover the benefits of biologics and education around the topic increases, there are hopes for the future of biologics.
Ethics of profitability in healthcare
The ethics of profitability in healthcare varies grossly across borders, taking the United States and the EU as examples – the United States has taken a capitalistic approach to healthcare, with high barriers to affordable treatment and even higher profit margins. Alternatively, European countries boast some of the most accessible healthcare in the world.
Biologic treatment solutions boast higher price tags than generics due to associated R&D costs, and patients can experience improved results; the current market does not currently highlight a practical solution for most patients. It is not without hope, since as the market settles, and so, prices do too, the barriers to accessibility of biologic treatments will change for the better. For now, businesses will either put the burden of cost on their profit margins until wider adoption takes place or keep the burden of cost on the patients in the name of profitability.
Conclusion
Biologics have the potential to surpass generics in popularity and usage, but it all boils down to cost efficiency and future technological advancements. There is no right answer to how biologics firms should structure their business models and pricing plans, but for now the burden of cost is being shouldered by the consumer until the tango between innovation and affordability settles into an equilibrium.








